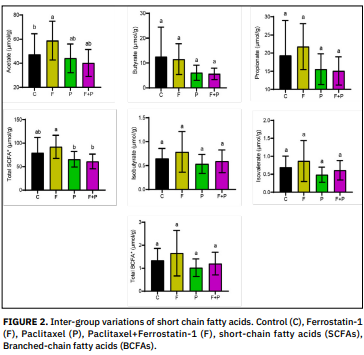
Effect of Paclitaxel and Ferrostatin-1 administration on fecal short chain fatty acids
Abstract
Short-chain fatty acids are organic acids manufactured by the gut microbiota. The type and amount of microflora in the colon, the source of substrate and the transition period through the intestine affect the rate and amount of Short-chain fatty acids production. Ferrostatin-1, a synthetic compound and a potent inhibitor of ferroptosis, is an antioxidant capable of inhibiting ferroptosis. Paclitaxel is a type of chemotherapy called taxane and causes peripheral neuropathy as a side effect of cancer treatment. In this study, we researched the effect of paclitaxel and ferrostatin-1 administration on fecal Short- chain fatty acids. For this purpose, rats were divided into four groups as control (n = 6), paclitaxel (n = 8), ferrostatin (n = 9) and paclitaxel + ferrostatin (n = 9). Paclitaxel (10 mg/kg) and ferrostatin-1 (5 mg/kg) were administered intraperitoneally once a week for 4 weeks. At the end of the experiment, the amounts of Short-chain fatty acids (acetate, propionate and butyrate) and branched-chain fatty acids (iso-butyrate and iso-valerate) in the feces were determined by gas chromatography. According to the results obtained, acetate level increased significantly (P < 0.05) in Ferrostatin-1 treated group compared to control group and total Short-chain fatty acids level increased significantly (P < 0.05) in Ferrostatin-1group compared to Ferrostatin-1 + Paclitaxel group. Although statistically insignificant, it was observed that ferrostatin-1 increased all the Short-chain fatty acids except butyrate, while paclitaxel decreased all the Short-chain fatty acids. The findings of this study suggest that ferrostatin-1 and paclitaxel may affect the functions of microorganisms in the large intestine and thus the amount of microbial Short-chain fatty acids. In addition, it is clear that therapeutic targeting of these specific bacteria, and thus the produced Short-chain fatty acids, will be important for successful treatment regimens and improved quality of life, especially in cancer patients, and may improve treatment outcomes.
Downloads
References
Akbarali HI, Muchhala KH, Jessup DK, Cheatham S. Chapter Four - Chemotherapy induced gastrointestinal toxicities. Adv. Cancer Res. [Internet]. 2022; 155:131166. doi: https://doi.org/hbcjxg
Hersi F, Elgendy SM, Al Shamma SA, Altell RT, Sadiek O, Omar HA. Cancer immunotherapy resistance: The impact of microbiome-derived short-chain fatty acids and other emerging metabolites. Life Sci. [Internet]. 2022; 300:120573. doi: https://doi.org/qjv2 DOI: https://doi.org/10.1016/j.lfs.2022.120573
Yang K, Li G, Li Q, Wang W, Zhao X, Shao N, Qiu H, Liu J, Xu L, Zhao J. Distribution of gut microbiota across intestinal segments and their impact on human physiological and pathological processes. Cell. Biosci. [Internet]. 2025; 15(1):47. doi: https://doi.org/g9j8h3 DOI: https://doi.org/10.1186/s13578-025-01385-y
Zhang D, Jian YP, Zhang YN, Li Y, Gu LT, Sun HH, Liu MD, Zhou HL, Wang YS, Xu ZX. Short-chain fatty acids in diseases. Cell Commun. Signal. [Internet]. 2023; 21(1):212. doi: https://doi.org/g2355t DOI: https://doi.org/10.1186/s12964-023-01219-9
Fusco W, Bernabeu-Lorenzo M, Cintoni M, Porcari S, Rinninella E, Kaitsas F, Lener E, Mele MC, Gasbarrini A, Collado MC, Cammarota G, Ianiro G. Short-chain fattyacid-producing bacteria: key components of the human gut microbiota. Nutrients. [Internet]. 2023; 15(9):2211. doi: https://doi.org/g8p99z DOI: https://doi.org/10.3390/nu15092211
Li S, Zhu S, Yu J. The role of gut microbiota and metabolites in cancer chemotherapy. J. Adv. Res. [Internet]. 2024; 64:223-235. doi: https://doi.org/gs86n9 DOI: https://doi.org/10.1016/j.jare.2023.11.027
Sun Y, Chen P, Zhai B, Zhang M, Xiang Y, Fang J, Xu S, Gao Y, Chen X, Sui X, Li G. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. [Internet]. 2020; 127:110108. doi: https://doi.org/ghq7np DOI: https://doi.org/10.1016/j.biopha.2020.110108
Yao T, Li L. The influence of microbiota on ferroptosis in intestinal diseases. Gut Microbes. [Internet]. 2023; 15(2):2263210. doi: https://doi.org/qjwf DOI: https://doi.org/10.1080/19490976.2023.2263210
Miotto G, Rossetto M, Di Paolo ML, Orian L, Venerando R, Roveri A, Vuckovic AM, Bosello-Travain V, Zaccarin M, Zennaro L, Maiorino M, Toppo S, Ursini F, Cozza G. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. [Internet]. 2020; 28:101328. doi: https://doi.org/gnm9qm DOI: https://doi.org/10.1016/j.redox.2019.101328
Wang G, Qin S, Chen L, Geng H, Zheng Y, Xia C, Yao J, Deng L. Butyrate dictates ferroptosis sensitivity through FFAR2-mTOR signaling. Cell Death Dis. [Internet]. 2023; 14(4):292. doi: https://doi.org/gs8nw8 DOI: https://doi.org/10.1038/s41419-023-05778-0
He Y, Ling Y, Zhang Z, Mertens RT, Cao Q, Xu X, Guo K, Shi Q, Zhang X, Huo L, Wang K, Guo H, Shen W, Shen M, Feng W, Xiao P. Butyrate reverses ferroptosis resistance in colorectal cancer by inducing c-Fos-dependent xCT suppression. Redox Biol. [Internet]. 2023; 65:102822. doi: https://doi.org/qjwj DOI: https://doi.org/10.1016/j.redox.2023.102822
Son MY, Cho HS. Anticancer effects of gut microbiotaderived short-chain fatty acids in cancers. J. Microbiol. Biotechnol. [Internet]. 2023; 33(7):849-856. doi: https://doi.org/qjwp DOI: https://doi.org/10.4014/jmb.2301.01031
Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. [Internet]. 2007; 6(5):609-621. doi: https://doi.org/d53z5f DOI: https://doi.org/10.1517/14740338.6.5.609
Ma Y, Yu S, Ni S, Zhang B, Kung ACF, Gao J, Lu A, Zhang G. Targeting strategies for enhancing paclitaxel specificity in chemotherapy. Front. Cell Dev. Biol. [Internet]. 2021; 9:626910. doi: https://doi.org/gk84v7 DOI: https://doi.org/10.3389/fcell.2021.626910
Plaza-Diaz J, Álvarez-Mercado AI. The interplay between microbiota and chemotherapy-derived metabolites in breast cancer. Metabolites. [Internet]. 2023; 13(6):703. doi: https://doi.org/qjws DOI: https://doi.org/10.3390/metabo13060703
Al-Qadami GH, Secombe KR, Subramaniam CB, Wardill HR, Bowen JM. Gut microbiota-derived short-chain fatty acids: impact on cancer treatment response and toxicities. Microorganisms. [Internet]. 2022; 10(10):2048. doi: https://doi.org/qjwv DOI: https://doi.org/10.3390/microorganisms10102048
Bishehsari F, Engen PA, Preite NZ, Tuncil YE, Naqib A, Shaikh M, Rossi M, Wilber S, Green SJ, Hamaker BR, Khazaie K, Voigt RM, Forsyth CB, Keshavarzian A. Dietary fiber treatment corrects the composition of gut microbiota, promotes SCFA production, and suppresses colon carcinogenesis. Genes. [Internet]. 2018; 9(2):102. doi: https://doi.org/gmbppr DOI: https://doi.org/10.3390/genes9020102
Lebet V, Arrigoni E, Amadò R. Measurement of fermentation products and substrate disappearance during incubation of dietary fibre sources with human faecal flora. LWT. [Internet]. 1998; 31(5):473-479. doi: https://doi.org/cnpqjb DOI: https://doi.org/10.1006/fstl.1998.0401
Tuncil YE, Nakatsu CH, Kazem AE, Arioglu-Tuncil S, Reuhs B, Martens EC, Hamaker BR. Delayed utilization of some fast-fermenting soluble dietary fibers by human gut microbiota when presented in a mixture. J. Funct. Foods. [Internet]. 2017; 32:347-357. doi: https://doi.org/f96kjd DOI: https://doi.org/10.1016/j.jff.2017.03.001
Stein A, Voigt W, Jordan K. Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther. Adv. Med. Oncol. [Internet]. 2010; 2(1):51-63. doi: https://doi.org/bqw264 DOI: https://doi.org/10.1177/1758834009355164
Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Keefe DM. Faecal microflora and ß-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats. Cancer Biol. Ther. [Internet]. 2008; 7(12):1919-1925. doi: https://doi.org/bz6gc3 DOI: https://doi.org/10.4161/cbt.7.12.6940
Loman BR, Jordan KR, Haynes B, Bailey MT, Pyter LM. Chemotherapy-induced neuroinflammation is associated with disrupted colonic and bacterial homeostasis in female mice. Sci. Rep. [Internet]. 2019; 9(1):16490. doi: https://doi.org/gsgbp3 DOI: https://doi.org/10.1038/s41598-019-52893-0
Loman BR, Alzoubi Z, Lynch AJ, Jaggers RM, Jordan K, Grant CV, Rogers LK, Pyter LM, Bailey MT. Paclitaxel chemotherapy disrupts microbiota-enterohepatic bile acid metabolism in mice. Gut Microbes. [Internet]. 2024; 16(1):2410475. doi: https://doi.org/qjw2 DOI: https://doi.org/10.1080/19490976.2024.2410475
Yuan M, Wang Y, Tian X, Zheng W, Zuo H, Zhang X, Song H. Ferrostatin-1 improves prognosis and regulates gut microbiota of steatotic liver transplantation recipients in rats. Future Microbiol. [Internet]. 2024; 19(5):413-429. doi: https://doi.org/qjw3 DOI: https://doi.org/10.2217/fmb-2023-0133
Dögüs Y, Deami A, Yönden Z. Mikrobiyota kaynakli kisa zincirli yag asitleri ve hastaliklar üzerine etkileri. Arsiv Kaynak Tarama Dergisi. [Internet]. 2023; 32(4):246-253. doi: https://doi.org/qjw4 DOI: https://doi.org/10.17827/aktd.1330297
Wang Z, Ma X, Shi W, Zhu W, Feng X, Xin H, Zhang Y, Cong B, Li Y. The gut microbiota metabolite butyrate modulates acute stress-induced ferroptosis in the prefrontal cortex via the gut–brain axis. Int. J. Mol. Sci. [Internet]. 2025; 26(4):1698. doi: https://doi.org/qjw6 DOI: https://doi.org/10.3390/ijms26041698
Wang X, Li W, Dong Y, Zhang Y, Huo Q, Lu L, Zhang J, Zhao Y, Fan S, Dong H, Li D. Ferrostatin-1 mitigates ionizing radiation-induced intestinal injuries by inhibiting apoptosis and ferroptosis: an in vitro and in vivo study. Int. J. Radiat. Biol. [Internet]. 2023; 99(10):1607-1618. doi: https://doi.org/hbbdc9 DOI: https://doi.org/10.1080/09553002.2023.2194399
Jessup D, Woods K, Thakker S, Damaj MI, Akbarali HI. Short-chain fatty acid butyrate prevents morphine- and paclitaxel-induced peripheral hypersensitivity. Sci. Rep. [Internet]. 2023; 13(1):17805. doi: https://doi.org/qjw7 DOI: https://doi.org/10.1038/s41598-023-44857-2
Cristiano C, Cuozzo M, Coretti L, Liguori FM, Cimmino F, Turco L, Avagliano C, Aviello G, Mollica MP, Lembo F, Russo R. Oral sodium butyrate supplementation ameliorates paclitaxel-induced behavioral and intestinal dysfunction. Biomed. Pharmacother. [Internet]. 2022; 153:113528. doi: https://doi.org/qjw8 DOI: https://doi.org/10.1016/j.biopha.2022.113528