Evaluation of the effectiveness of local Melatonin applied at different doses on healing of bone defect in rat tibias
Abstract
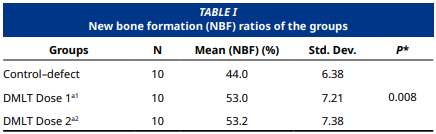
This study aimed to investigate the effect of local melatonin application on bone healing in critical size defects created in rat tibias. 30 Spraque Dawley rats selected for the experimental setup were divided into three groups, each with 10 rats. In the control– defect group (n=10), only defects were created in the tibia bones of the rats, while 1.2 mg and 3 mg melatonin were applied locally to the defect–melatonin dose 1 (n=10) and defect melatonin dose 2 (n=10) groups, respectively. At the end of the experiment the subjects in all the groups were euthanized after an 8-week healing period and defect healing was calculated with histological bone healing percentage. The Shapiro–Wilk test and the Kolmogorov–Smirnov test were used to evaluate the conformity of the data to a normal distribution. One–way ANOVA was used to determine whether there was a difference between the groups due to the normal distribution of the data, and Tukey’s honestly significant difference (HSD) test was used in pairwise comparisons to determine from which group the difference originated. The healing rate in the defect melatonin dose 2 group was calculated as 53.2 ± 7.38% and the healing rate in the control group was calculated as 44 ± 6.38%, which was a significant increase (P=0.008); however, when the difference between the defect melatonin dose 1 group (53 ± 7.1%) and the defect melatonin dose 2 group (53. 2± 7.38%) was evaluated statistically, no significant difference was found.The bone healing percentage in the groups where local melatonin was applied was found to be statistically significantly higher compared to the control group (P<0.05). No statistically significant difference was found between the groups where local melatonin was applied in terms of bone healing percentage (P>0.05). Within the limits of this study, it can be said that local melatonin application applied at two different doses had a positive effect on bone healing, however, no difference was observed between the applied local melatonin doses in terms of bone healing.
Downloads
References
Dorea HC, McLaughlin RM, Cantwell HD, Read R, Armbrust L, Pool R, Roush JK, Boyle C. Evaluation of healing in feline femoral defects filled with cancellous autograft, cancellous allograft or Bioglass. Vet. Comp. Orthop. Traumatol. 2005; 18(3):157-168. PMID: 16594447. DOI: https://doi.org/10.1055/s-0038-1632947
Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS. The cell biology of bone metabolism. J. Clin. Pathol. [Internet]. 2008; 61(5):577-587. doi: https://doi.org/fjm8vr DOI: https://doi.org/10.1136/jcp.2007.048868
Hadjidakis DJ, Androulakis II. Bone remodeling. Ann. NY. Acad. Sci. [Internet]. 2006; 1092:385-396. doi: https://doi.org/fr69hb DOI: https://doi.org/10.1196/annals.1365.035
Tanrisever M, Eröksüz H, Bulut S. The comparison of the effects of intraarticular injections of bovine amniotic fluid and hyaluronic acid on cartilage tissue in an experimental osteoarthritic rabbit model: histopathological and immunohistochemical results. Turk J. Vet. Anim. Sci. [Internet]. 2017; 41:273-228. doi: https://doi.org/mhsj DOI: https://doi.org/10.3906/vet-1605-107
Global Burden of Disease (GBD). Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of disease study 2019. Lancet Healthy Longev. [Internet]. 2021; 2(9):e580-e592. doi: https://doi.org/gpkknk DOI: https://doi.org/10.1016/S2215-0366(21)00395-3
Irie K, Alpaslan C, Takahashi K, Kondo Y, Izumi N, Sakakura Y, Tsuruga E, Nakajima T, Ejiri S, Ozawa H, Yajima T. Osteoclast differentiation in ectopic bone formation induced by recombinant human bone morphogenetic protein 2 (rhBMP-2). J. Bone Miner. Metab. [Internet]. 2003; 21(6):363-369. doi: https://doi.org/dpshf8 DOI: https://doi.org/10.1007/s00774-003-0430-x
Wang M, Weng YL, Hu XJ, Zhang Y, Chai G, Zhu L, Liu W, Cui L, Feng XP, Cao YL. [Repair of alveolar bone defect with tissue engineered bone: an experimental study of dogs]. Zhonghua Yi Xue Za Zhi. [Internet]. 2003 [cited Mar 15, 2025]; 83(15):1339-1344. Chinese. PMID: 12930691. Available in: https://goo.su/vKHqRa
Cajochen C, Kräuchi K, Wirz–Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J. Neuroendocrinol. [Internet]. 2003; 15(4):432-437. doi: https://doi.org/b7x76n DOI: https://doi.org/10.1046/j.1365-2826.2003.00989.x
Luo B, Zhou X, Tang Q, et al. Circadian rhythms affect bone reconstruction by regulating bone energy metabolism. J. Transl. Med. [Internet]. 2021; 19(1):410. doi: https://doi.org/p584 DOI: https://doi.org/10.1186/s12967-021-03068-x
Ostrin LA. Ocular and systemic melatonin and the influence of light exposure. Clin. Exp. Optom. [Internet]. 2019; 102(2):99-108. doi: https://doi.org/gn24qt DOI: https://doi.org/10.1111/cxo.12824
Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine [Internet]. 2005; 27(2):101–110. doi: https://doi.org/bbgmf7 DOI: https://doi.org/10.1385/ENDO:27:2:101
Roth JA, Kim BG, Lin WL, Cho MI. Melatonin promotes osteoblast differentiation and bone formation. J. Biol. Chem. [Internet]. 1999; 274(31):22041-22047. doi: https://doi.org/b78n4z DOI: https://doi.org/10.1074/jbc.274.31.22041
Lin GJ, Huang SH, Chen SJ, Wang CH, Chang DM, Sytwu HK. Modulation by melatonin of the pathogenesis of inflammatory autoimmune diseases. Int. J. Mol. Sci. [Internet]. 2013; 14(6):11742-11766. doi: https://doi.org/f4z8w5 DOI: https://doi.org/10.3390/ijms140611742
Zheng S, Zhou C, Yang H, Li J, Feng Z, Liao L, Li Y. Melatonin accelerates osteoporotic bone defect repair by promoting osteogenesis–angiogenesis coupling. Front. Endocrinol. [Internet]. 2022; 13:826660. doi: https://doi.org/p6bt DOI: https://doi.org/10.3389/fendo.2022.826660
Dundar S, Yaman F, Saybak A, et al. Evaluation of effects of topical melatonin application on osseointegration of dental implant: An experimental study. J. Oral Implantol. [Internet]. 2016; 42(5):386-389. doi: https://doi.org/f9d8mf DOI: https://doi.org/10.1563/aaid-joi-D-16-00048
Satomura K, Tobiume S, Tokuyama R, et al. Melatonin at pharmacological doses enhances human osteoblastic differentiation in vitro and promotes mouse cortical bone formation in vivo. J. Pineal Res. [Internet]. 2007; 42(3):231-239. doi: https://doi.org/bxhxg7 DOI: https://doi.org/10.1111/j.1600-079X.2006.00410.x
Reiter RJ. Melatonin: clinical relevance. Best Pract. Res. Clin. Endocrinol. Metab. [Internet]. 2003; 17(2):273-285. doi: https://doi.org/fhj8qk DOI: https://doi.org/10.1016/S1521-690X(03)00016-2
Muñoz F, López–Peña M, Miño N, Gómez–Moreno G, Guardia J, Cutando A. Topical application of melatonin and growth hormone accelerates bone healing around dental implants in dogs. Clin. Implant. Dent. Relat. Res. [Internet]. 2012; 14(2):226-235. doi: https://doi.org/cmwzwc DOI: https://doi.org/10.1111/j.1708-8208.2009.00242.x
Chen W, Chen X, Chen AC, Shi Q, Pan G, Pei M, Yang H, Liu T, He F.. Melatonin restores the osteoporosis–impaired osteogenic potential of bone marrow mesenchymal stem cells by preserving SIRT1-mediated intracellular antioxidant properties. Free Radic. Biol. Med. [Internet]. 2020; 146:92-106. doi: https://doi.org/p6bx DOI: https://doi.org/10.1016/j.freeradbiomed.2019.10.412
Zhou MS, Tao ZS. Systemic administration with melatonin in the daytime has a better effect on promoting osseointegration of titanium rods in ovariectomized rats. Bone Joint Res. [Internet]. 2022; 11(11):751-762. doi: https://doi.org/p6bz DOI: https://doi.org/10.1302/2046-3758.1111.BJR-2022-0017.R2
Cutando A, Arana C, Gómez–Moreno G, Escames G, López A, Ferrera MJ, Reiter RJ, Acuña–Castroviejo D. Local application of melatonin into alveolar sockets of beagle dogs reduces tooth removal–induced oxidative stress. J. Periodontol. [Internet]. 2007; 78(3):576-583. doi: https://doi.org/fpgwd2 DOI: https://doi.org/10.1902/jop.2007.060244
Zhang Y, Liu T, Yang H, He F, Zhu X. Melatonin: A novel candidate for the treatment of osteoarthritis. Aging. Res. Rev. [Internet]. 2022; 78:101635. doi: https://doi.org/p6b4 DOI: https://doi.org/10.1016/j.arr.2022.101635
Nehela Y, Killiny N. Melatonin is involved in citrus response to the pathogen huanglongbing via modulation of phytohormonal biosynthesis. Plant Physiol. [Internet]. 2020; 184(4):2216-2239. doi: https://doi.org/gp984g DOI: https://doi.org/10.1104/pp.20.00393
Sun T, Li J, Xing HL, Tao ZS, Yang M. Melatonin improves the osseointegration of hydroxyapatite–coated titanium implants in senile female rats. Z. Gerontol. Geriatr. [Internet]. 2020; 53(8):770-777. doi: https://doi.org/p6b5 DOI: https://doi.org/10.1007/s00391-019-01640-1
Takechi M, Tatehara S, Satomura K, Fujisawa K, Nagayama M. Effect of FGF-2 and melatonin on implant bone healing: a histomorphometric study. J. Mater Sci. Mater. Med. [Internet]. 2008; 19(8):2949-2952. doi: https://doi.org/fws83x DOI: https://doi.org/10.1007/s10856-008-3416-3
Acikan I, Mehmet G, Artas G, Yaman F, Deniz G, Bulmus O, Kom M, Kirtay M, Dundar S. Systemic melatonin application increases bone formation in mandibular distraction osteogenesis. Braz. Oral Res. [Internet]. 2018; 32:e85. doi: https://doi.org/gfch8t DOI: https://doi.org/10.1590/1807-3107bor-2018.vol32.0085
Virto L, Haugen HJ, Fernández–Mateos P, Cano P, González J, Jiménez–Ortega V, Esquifino AI, Sanz M. Melatonin expression in periodontitis and obesity: An experimental in vivo investigation. J. Periodontal Res. [Internet]. 2018; 53(5):825-831. doi: https://doi.org/gmt5h4 DOI: https://doi.org/10.1111/jre.12571
Shino H, Hasuike A, Arai Y, Honda M, Isokawa K, Sato S. Melatonin enhances vertical bone augmentation in rat calvaria secluded spaces. Med. Oral Patol. Oral. Cir. Bucal. [Internet]. 2016; 21(1):e122-e126. doi: https://doi.org/p6b6 DOI: https://doi.org/10.4317/medoral.20904
Chan YH, Ho KN, Lee YC, Chou MJ, Lew WZ, Huang HM, Lai PC, Feng SW. Melatonin enhances osteogenic differentiation of dental pulp mesenchymal stem cells by regulating MAPK pathways and promotes the efficiency of bone regeneration in calvarial bone defects. Stem Cell Res. Ther. [Internet]. 2022; 13(1):73. doi: https://doi.org/p6b7 DOI: https://doi.org/10.1186/s13287-022-02744-z
Wang B, Wen H, Smith W, Hao D, He B, Kong L. Regulation effects of melatonin on bone marrow mesenchymal stem cell differentiation. J. Cell Physiol. [Internet]. 2019; 234(2):1008- 1015. doi: https://doi.org/p6b8 DOI: https://doi.org/10.1002/jcp.27090
Costa KLD, Abreu LF, Tolomei CB, Eleutério RG, Basting R, Balbinot G, Collares FM, Lopes P, Veiga N, Fernandes GVO, Peruzzo DC. Use of local melatonin with xenogeneic bone graft to treat critical–size bone defects in rats with osteoporosis: A randomized study. J. Funct. Biomater. [Internet]. 2024; 15(5):124. doi: https://doi.org/g9dx45 DOI: https://doi.org/10.3390/jfb15050124
Bagherifard A, Hosseinzadeh A, Koosha F, Sheibani M, Karimi– Behnagh A, Reiter RJ, Mehrzadi S. Melatonin and bone–related diseases: an updated mechanistic overview of current evidence and future prospects. Osteoporos. Int. [Internet]. 2023; 34(10):1677-1701. doi: https://doi.org/p6cr DOI: https://doi.org/10.1007/s00198-023-06836-1
Liu L, Xu Y, Reiter RJ. Melatonin inhibits the proliferation of human osteosarcoma cell line MG-63. Bone. [Internet]. 2013; 55(2):432-438. doi: https://doi.org/f4236s DOI: https://doi.org/10.1016/j.bone.2013.02.021
Wang YP, Yang ZP. Effects of melatonin combined with Cis– platinum or methotrexate on the proliferation of osteosarcoma cell line SaOS-2. Acta Acad. Med. Sin. [Internet]. 2015; 37(2):215-220. doi: https://doi.org/p6cv
Altındal DÇ, Gümüşderelioğlu M. Melatonin releasing PLGA micro/nanoparticles and their effect on osteosarcoma cells. J. Microencapsul. [Internet]. 2016; 33(1):53-63. doi: https://doi.org/gqzn2j DOI: https://doi.org/10.3109/02652048.2015.1115901