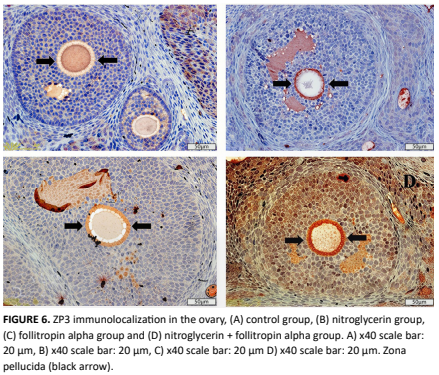
Demonstration of ovarian and brain tissue interactions as a result of stimulation of oogenesis in Migraine Modeled rats.
Abstract
Migraine, is a primary neurological disease. Although there is no gender difference in the prepubertal period, migraine is more common in adult women than in adult men. This situation decreases with menopause, but still its dominance in women continues. In our study, it is aimed to determine whether follitropin alfa could have a developmental benefit on the ovarian follicles of animals in migraine model after creating experimental migraine model with nitroglycerin on rats. In this study, the animals were divided into a total of four groups: a control group and three experimental groups. The first experimental group animals were injected with nitroglycerin, the other groups were injected with follitropin alfa and follitropin alfa together with nitroglycerin. At the end of 21 days, euthanasia was provided with pentothal sodium. Immunohistochemical methods were applied to post-mortem brain and ovarian tissues of rats. c-Fos was used as a migraine marker, Zona Pellucida 3 was used to show changes in the zona pellucida on the ovarian surface, MMP-9 and transient receptor potential vanilloid 1 were used to elucidate the pathogenesis of migraine. In our study, samples were evaluated by comparison with immunohistochemical staining. In animals, high c-Fos localization in the brain stem, high expression of Matrix metalloproteinase-9 in granular neurons, transient receptor potential vanilloid 1 in theca layer and Zona Pellucida 3 in the zona pellucida region were detected. The expression relationships of c-Fos, Matrix metalloproteinase-9, transient receptor potential vanilloid 1 and Zona Pellucida 3 in the brain stem, brain frontal cortex and ovary tissues where migraine and follitropin alfa were studied together have been shown for the first time in this study.
Downloads
References
Lisicki M, Schoenen J. Metabolic treatments of migraine. Expert. Rev. Neurother. [Internet]. 2020; 20(3):295-302 doi: https://doi.org/gk7wrw DOI: https://doi.org/10.1080/14737175.2020.1729130
Goadsby P, Holland P, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. [Internet]. 2017; 97(2):553-622. doi: https://doi.org/gh5s8v DOI: https://doi.org/10.1152/physrev.00034.2015
Costa D, Benincasa G, Lucchese R, Infante T, Nicoletti GF, Napoli C. Effect of nitric oxide reduction on arterial thrombosis. Scand. Cardiovasc. J. [Internet]. 2019; 53(1):1–8. doi: https://doi.org/ghkck6 DOI: https://doi.org/10.1080/14017431.2019.1581943
Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. [Internet]. 2014; 155(2):269–274. doi: https://doi.org/f5pb7p DOI: https://doi.org/10.1016/j.pain.2013.10.004
Stratford JM, Thompson JA. MSG-Evoked c-Fos activity in the nucleus of the solitary tract is dependent upon fluid delivery and stimulation parameters. Chem. Senses. [Internet]. 2016; 41(3):211–220. doi: https://doi.org/f8dnwd DOI: https://doi.org/10.1093/chemse/bjv082
Maleki N, Androulakis XM. Is there any MRI pattern that discriminates female from male migraine patients? Fro. nt Neurol. [Internet]. 2019; 10:961. doi: https://doi.org/g9hc9j DOI: https://doi.org/10.3389/fneur.2019.00961
Yan Z, Dai Y, Fu H, Zheng Y, Bao D, Yin Y, Chen Q, Nie X, Hao Q, Hou D, Cui Y. Curcumin exerts a protective effect against premature ovarian failure in mice.J. Mol. Endocrinol. [Internet]. 2018;60(3):261-271. doi: https://doi.org/gc7dmj DOI: https://doi.org/10.1530/JME-17-0214
Dong C, Ye DX, Zhang WB, Pan HY, Zhang ZY, Zhang L. Overexpression of c-fos promotes cell invasion and migration via CD44 pathway in oralsquamous cell carcinoma. J. Oral Pathol. Med. [Internet]. 2015; 44(5):353-360. doi: https://doi.org/qpv9 DOI: https://doi.org/10.1111/jop.12296
Vafadari B, Salamian A, Kaczmarek L. MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J. Neurochem. [Internet]. 2016; 139(S2):91- DOI: https://doi.org/10.1111/jnc.13415
Feng J, Yang P, Mack MR, Dryn D, Luo J, Gong X, Liu S, Oetjen LK, Zholos AV, Mei Z, Yin S, Kim BS, Hu H. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. [Internet]. 2017; 8:980. doi: https://doi.org/gchz82 DOI: https://doi.org/10.1038/s41467-017-01056-8
Litscher ES, Wassarman PM. Zona Pellucida Proteins, Fibrils and Matrix. Annu. Rev. Biochem. [Internet]. 2020; 89:695-715. doi: https://doi.org/gn8ktc
Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu. Rev. Physiol. [Internet]. 2013; 75:365–391. doi: https://doi.org/gm4h2n DOI: https://doi.org/10.1146/annurev-physiol-030212-183717
Yang DG, Gao YY, Yin ZQ, Wang XR, Meng XS, Zou TF, Duan YJ, Chen YL, Liao CZ, Xie ZL, Fan XD, Sun L, Han JH, Yang XX. Roxadustat alleviates nitroglycerin-induced migraine in mice by regulating HIF-1α/NF-κB/inflammation pathway. Acta Pharmacol. Sin. [Internet]. 2023; 44(2):308-320. doi: https://doi.org/qpwc DOI: https://doi.org/10.1038/s41401-022-00941-3
Pacchiarotti A, Selman H, Valeri C, Napoletano S, Sbracia M, Antonini G, Biagiotti G, Pacchiarotti A. Ovarian Stimulation Protocol in IVF: An Up-to-Date Review of the Literature. Curr. Pharm. Biotechnol. [Internet]. 2016; 17(4):303–315. doi: https://doi.org/qpwd DOI: https://doi.org/10.2174/1389201017666160118103147
Velazquez FN, Caputto BL, Boussin FD. c-Fos importance for brain development. Aging (Albany NY). [Internet]. 2015; 7(12):1028-1029. doi: https://doi.org/qpwf DOI: https://doi.org/10.18632/aging.100862
Rodriguez C, Agulla J, Delgado-Esteban M. Refocusing the Brain: New Approaches in Neuroprotection Against Ischemic Injury. Neurochem. Res. [Internet]. 2021; 46(1):51-63. doi: https://doi.org/qpwg DOI: https://doi.org/10.1007/s11064-020-03016-z
Kaler S, Dhar P, Bhattacharya A, Mehra RD. Preliminary morphological and immunohistochemical changes in rat hippocampus following postnatal exposure to sodium arsenite. Toxicol. Int. [Internet]. 2013; 20(2):160-169. doi: https://doi.org/qpwh DOI: https://doi.org/10.4103/0971-6580.117259
Aghazadeh-Tabrizi M, Baraldi PG, Baraldi S, Gessi S, Merighi S, Borea PA. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists. Med. Res. Rev. [Internet]. 2017; 37(4):936-983. doi: https://doi.org/f9g7t3 DOI: https://doi.org/10.1002/med.21427
Li F, Wang F. TRPV1 in Pain and Itch. In: Zhou, L. (eds). Ion Channels in Biophysics and Physiology. Advances in Experimental Medicine and Biology. Singapore: Springer. [Internet]. 2021; 1349:249-273. doi: https://doi.org/gtn83j DOI: https://doi.org/10.1007/978-981-16-4254-8_12
Kolesár D, Kolesárová M, Kyselovič J. Distribution pattern of dorsal root ganglion neurons synthesizing nitric oxide synthase in different animal species. Can. J. Physiol. Pharmacol. [Internet]. 2017; 95(4):328-332. doi: https://doi.org/qpwj DOI: https://doi.org/10.1139/cjpp-2016-0294
Iglesias LP, Aguiar DC, Moreira FA. TRPV1 blockers as potential new treatments for psychiatric disorders. Behav. Pharmacol. [Internet]. 2022; 33(1):2-14. doi: https://doi.org/qpwk DOI: https://doi.org/10.1097/FBP.0000000000000603
Ren M, Wang T, Huang L, Ye X, Xv Z, Ouyang C, Han Z. Role of VR1 in the differentiation of bone marrow- derived mesenchymal stem cells into cardiomyocytes associated with Wnt/β-catenin signaling. Cardiovasc. Ther. [Internet]. 2016; 34(6):482-488. doi: https://doi.org/f9chpp DOI: https://doi.org/10.1111/1755-5922.12228
Wassarman PM, Litscher ES. The Mouse Egg’s Zona Pellucida. Curr. Top. Dev. Biol. [Internet]. 2018; 130:331-356. doi: https://doi.org/qpwm DOI: https://doi.org/10.1016/bs.ctdb.2018.01.003
Litscher ES, Wassarman PM. Zona Pellucida Proteins, Fibrils, and Matrix. Annu. Rev. Biochem. [Internet]. 2020; 89:695-715. doi: https://doi.org/gn8ktc DOI: https://doi.org/10.1146/annurev-biochem-011520-105310