Efecto de Lavandula stoechas y peróxido de hidrógeno en codornices sobre la producción de huevos, a nivel hepatico, sanguíneo y expresión genética
Resumen
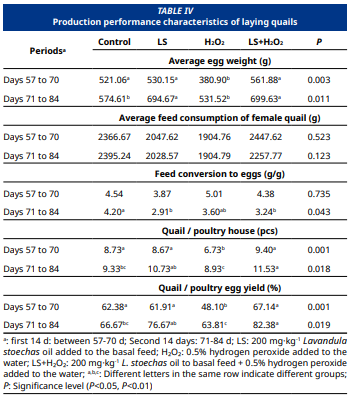
El presente estudio tuvo como propósito evaluar los efectos de la suplementación con aceite de Lavandula stoechas (LSO) en el alimento y peróxido de hidrógeno (H₂O₂) en el agua de consumo sobre la producción de huevos, la incubabilidad y las características de la canal en codornices de entre 56 y 83 días (d) de edad durante el período de puesta. Al mismo tiempo, se analizaron los niveles de expresión de los genes GPx7 y NRF2, así como parámetros bioquímicos del suero sanguíneo y características histopatológicas del hígado, con el fin de determinar si la suplementación con LSO ejerce un efecto antioxidante en codornices expuestas a H2O2. En el estudio, se utilizaron 84 codornices, distribuidas en grupos compuestos por 15 hembras y 6 machos cada uno. Se establecieron cuatro grupos: un grupo control con alimento basal; un grupo LS con 200 mg·kg-1 de LSO añadido al alimento basal; un grupo H2O2 con alimento basal + 0,5 % de H2O2 en el agua; y un grupo LS + H2O2 con alimento basal + 200 mg·kg-1 de LSO + 0,5 % de H2O2 en el agua. Cada grupo fue replicado tres veces. Durante los segundos 14 d (días 71 a 84), tanto el grupo control como el grupo con H2O2 presentaron un menor peso promedio de los huevos (P<0,05) en comparación con los grupos LS y LS + H2O2. Además, los grupos LS y LS+H2O2 mostraron una mejor eficiencia de conversión alimenticia en comparación con el grupo control (P<0,05). Asimismo, la tasa de fertilización y el rendimiento de incubación fueron más altos en los grupos suplementados con LSO en comparación con el grupo control (P>0,05). Por el contrario, el grupo al que se añadió H2O2 en el agua mostró rendimientos más bajos que los demás grupos. Asimismo, se constató que el LSO incrementó la expresión del gen GPx7 en ambos sexos, indicando un posible efecto antioxidante, aunque no se detectaron cambios significativos en los niveles de expresión del gen NRF2.
Descargas
Citas
Santos TC, Murakami AE, Fanhani JC, Oliveira CAL. Production and reproduction of egg – and meattype quails reared in different group sizes. Braz. J. Poult. Sci. [Internet]. 2011; 13(1):09–14. doi: https://doi.org/dzfh45 DOI: https://doi.org/10.1590/S1516-635X2011000100002
Tolik D, Polawska E, Charuta A, Nowaczewski S, Cooper R. Characteristics of egg parts, chemical composition and nutritive value of Japanese quail eggs–a review. Folia Biol. [Internet]. 2014; 62(4):287–292. doi: https://doi.org/p4zc DOI: https://doi.org/10.3409/fb62_4.287
Shi W, Liu L, Li J, Qu L, Pang X, Yu H, Zhang Y, Wang T. Bioactive flavonoids from Flos Sophorae. J. Nat. Med. [Internet]. 2017; 71(3):513–522. doi: https://doi.org/p4zd DOI: https://doi.org/10.1007/s11418-017-1084-7
Hargis PS, Van Elswyk ME. Manipulating the fatty acid composition of poultry meat and eggs for the health conscious consumer. J. World’s Poult. Sci. [Internet]. 1993; 49(3):251–264. doi: https://doi.org/d83hkp DOI: https://doi.org/10.1079/WPS19930023
Tan Z, Halter B, Liu D, Gilbert ER, Cline MA. Dietary flavonoids as modulators of lipid metabolism in poultry. Front. Physiol. [Internet]. 2022; 13:863860. doi: https://doi.org/p4zf DOI: https://doi.org/10.3389/fphys.2022.863860
Giray ES, Kirici S, Kaya DA, Türk M, Sönmez Ö, İnan M. Comparing the effect of sub–critical water extraction with conventional extraction methods on the chemical composition of Lavandula stoechas. Talanta [Internet]. 2008; 74(4):930– 935. doi: https://doi.org/cck7hz DOI: https://doi.org/10.1016/j.talanta.2007.07.040
Kaplan EN, Çaki MN, Paşa S. Lavandula stoechas (karabaş otu) bitki özütünden kozmetik ürün eldesi. [Obtaining cosmetic products from Lavandula stoechas plant extract]. UMÜFED Uluslararası Batı Karadeniz Mühendislik ve Fen Bilim. Derg. [Internet]. 2019 [cited Apr 12, 2025]; 1(1):24–37. Turkish. Available in: https://goo.su/4ehdca
Gülçin Ì, Şat İG, Beydemir Ş, Elmastaş M, Küfrevioǧlu Öİ. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem. [Internet]. 2004; 87(3):393–400. doi: https://doi.org/fmsmvs DOI: https://doi.org/10.1016/j.foodchem.2003.12.008
Cherrat L, Espina L, Bakkali M, Pagán R, Laglaoui A. Chemical composition, antioxidant and antimicrobial properties of Mentha pulegium, Lavandula stoechas and Satureja calamintha Scheele essential oils and an evaluation of their bactericidal effect in combined processes. Innov. Food Sci. Emerg. Technol. [Internet]. 2014; 22:221–229. doi: https://doi.org/f52jkr DOI: https://doi.org/10.1016/j.ifset.2013.12.016
Özcan O, Erdal H, Çakirca G, Yönden Z. Oxidative stress and its impacts on intracellular lipids, proteins and DNA. J. Clin. Exp. Invest. [Internet]. 2015; 6(3):331–336. doi: https://doi.org/p4zh DOI: https://doi.org/10.5799/ahinjs.01.2015.03.0545
Kűçűkgűl A, İşgőr MM, Önel SE. Investigation of the bioactivity of escin in hydrogen peroxide–induced oxidative stress. J. Hell. Vet. Med. Soc. [Internet]. 2023; 74(2):5821–5828. doi: https://doi.org/pgms DOI: https://doi.org/10.12681/jhvms.30408
Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. [Internet]. 2004; 55:373–399. doi: https://doi.org/cdrqh7 DOI: https://doi.org/10.1146/annurev.arplant.55.031903.141701
Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. [Internet]. 2005; 17(7):1866–1875. doi: https://doi.org/bwwdhr DOI: https://doi.org/10.1105/tpc.105.033589
Ma Y, Zhao L, Gao M, Loor JJ. Tea polyphenols protect bovine mammary epithelial cells from hydrogen peroxide–induced oxidative damage in vitro. J. Anim. Sci. [Internet]. 2018; 96(10):4159–4172. doi: https://doi.org/gfdmqb DOI: https://doi.org/10.1093/jas/sky278
Mousa YJ, Amin SM, Shaaban KHA. Pharmacokinetics and plasma concentration of thiopental in normal and stressed chickens with hydrogen peroxide. J. Hell. Vet. Med. Soc. [Internet]. 2021 [cited Mar 04, 2025]; 72(2):2961–2968. Available in: https://goo.su/hF1rs
National Research Council (NRC). Nutrient Requirements of Poultry. 9th Rev. Washington, DC.(USA): National Academy Press. 1994.
Li S, Zheng X, Zhang X. Yu H, Han B, Yueying Lv, Liu Y, Wang X, Zhang Z. Exploring the liver fibrosis induced by deltamethrin exposure in quails and elucidating the protective mechanism of resveratrol. Ecotoxicol. Environ. Saf. [Internet]. 2021; 207:111501. doi: https://doi.org/ghv23z DOI: https://doi.org/10.1016/j.ecoenv.2020.111501
Bastos MS, Del Vesco AP, Santana TP, Santos TS, de Oliveira Junior GM, Fernandes RPM, Barbosa LT, Gasparion E. The role of cinnamon as a modulator of the expression of genes related to antioxidant activity and lipid metabolism of laying quails. PloS One. [Internet]. 2017; 12(12):e0189619. doi: https://doi.org/p4zm DOI: https://doi.org/10.1371/journal.pone.0189619
Ji YL, Wang H, Zhao XF, Wang Q, Zhang C, Zhang Y, Zhao M, Chen YH, Meng XH, Xu DX. Crosstalk between endoplasmic reticulum stress and mitochondrial pathway mediates cadmium–induced germ cell apoptosis in testes. Toxicol. Sci. [Internet]. 2011; 124(2):446–459. doi: https://doi.org/bkwxsk DOI: https://doi.org/10.1093/toxsci/kfr232
Güvenç M, Cellat M, Gökçek İ, Özkan H, Arkali G, Yakan A, Özsoy ŞY, Aksakal M. Nobiletin attenuates acetaminophen induced hepatorenal toxicity in rats. J. Biochem. Mol. Toxicol. [Internet]. 2020;34(2):e22427. doi: https://doi.org/mmw5 DOI: https://doi.org/10.1002/jbt.22427
Özkan H, Kutlu T, Yakin A, Özsoy ŞY. Molecular, biochemical and histopathological effects of long term low and high percentage fructose consumption on liver in rats. Ank. Univ. Vet. Fak. Derg. [Internet]. 2022; 69(4):409–417. doi: https://doi.org/mmw6 DOI: https://doi.org/10.33988/auvfd.855124
Uygun M, Kilimci N, Küçük Kaya S, Yavaş İ. Investigation of some chemical and biochemical properties of locally grown L. stoechas cariensis. Celal Bayar Univ. J. Sci. [Internet]. 2017; 13(1):63–69. doi: https://doi.org/p4zq DOI: https://doi.org/10.18466/cbayarfbe.302643
Küçükyilmaz K, Kiyma Z, Akdağ A, Çetinkaya M, Atalay H, Ateş A, Gürsel FE, Bozkurt M. Effect of lavender (Lavandula stoechas) essential oil on growth performance, carcass characteristics, meat quality and antioxidant status of broilers. S. Afr. J. Anim. Sci. [Internet]. 2017; 47(2):178–186. doi: https://doi.org/f9v4p8 DOI: https://doi.org/10.4314/sajas.v47i2.9
Laghouati O, Arbouche F, Arbouche Y. Effects of using essential oil of Lavandula stoechas in quail feed on growth performance, carcass characteristics, meat quality, and health status. Vet. World. [Internet]. 2020; 13(4):789–795. doi: https://doi.org/p4zr DOI: https://doi.org/10.14202/vetworld.2020.789-795
Torki M, Mohebbifar A , Mohammadi H. Effects of supplementing hen diet with Lavandula angustifolia and/or Mentha spicata essential oils on production performance, egg quality and blood variables of laying hens. Vet. Med. Sci. [Internet]. 2021; 7(1):184–193. doi: https://doi.org/gnjhsb DOI: https://doi.org/10.1002/vms3.343
Özbilgin A, Kara K. Effect of adding lavender oil to laying quail diets on performance, egg quality, oxidative status, and fatty acid profile. Trop. Anim. Health Prod. [Internet]. 2023; 55(3):173. doi: https://doi.org/p4zv DOI: https://doi.org/10.1007/s11250-023-03596-2
Williams P, Losa R. The use of essential oils and their compounds in poultry nutrition. World’s Poult. Sci. J. 2001; 17:14–15.
Radwan Nadia L, Hassan RA, Qota EM, Fayek HM. Effect of natural antioxidant on oxidative stability of eggs and productive and reproductive performance of laying hens. Int. J. Poult. Sci. [Internet]. 2008; 7(2):134–150. doi: https://doi.org/fq947h DOI: https://doi.org/10.3923/ijps.2008.134.150
Scholtz N, Halle I, Flachowsky G, Sauerwein H. Serum chemistry reference values in adult Japanese quail (Coturnix coturnix japonica) including sex–related differences. Poult. Sci. [Internet]. 2009; 88(6):1186–1190. doi: https://doi.org/b93fh5 DOI: https://doi.org/10.3382/ps.2008-00546
Alonso–Alvarez C, Pérez C, Velando A. Effects of acute exposure to heavy fuel oil from the Prestige spill on a seabird. Aquat. Toxicol. [Internet]. 2007; 84(1):103–110. doi: https://doi.org/cxdkfd DOI: https://doi.org/10.1016/j.aquatox.2007.06.004
Oladokun S, MacIsaac J, Rathgeber B, Adewole D. Essential oil delivery route: effect on broiler chicken’s growth performance, blood biochemistry, intestinal morphology, immune, and antioxidant status. Animals [Internet]. 2021; 11(12):3386. doi: https://doi.org/p4zw DOI: https://doi.org/10.3390/ani11123386
Sahinler SŞ, Yilmaz BS, Sarikurkcu C, Tepe B. The importance of Lavandula stoechas L. in pharmacognosy and phytotherapy. Int. J. Second. Metab. [Internet]. 2022; 9(3):360–376. doi: https://doi.org/p4zx DOI: https://doi.org/10.21448/ijsm.1098975
Benabdelkader T, Zitouni A, Guitton Y, Jullien F, Maitre D, Casabianca H, Legendre L, Kameli A. Essential oils from wild populations of Algerian Lavandula stoechas L.: composition, chemical variability, and in vitro biological properties. Chem. Biodivers. [Internet]. 2011; 8(5):937–953. doi: https://doi.org/c5f7rt DOI: https://doi.org/10.1002/cbdv.201000301
Selmi S, Jallouli M, Gharbi N, Marzouki L. Hepatoprotective and renoprotective effects of lavender (Lavandula stoechas L.) essential oils against malathion–induced oxidative stress in young male mice. J. Med. Food [Internet]. 2015; 18(10):1103– 1111. doi: https://doi.org/p4zz DOI: https://doi.org/10.1089/jmf.2014.0130
Mahgoub SAM, Abd El–Hack ME, Saadeldin IM, Hussein MA, Swelum AA, Alagawany M. Impact of Rosmarinus officinalis cold–pressed oil on health, growth performance, intestinal bacterial populations, and immunocompetence of Japanese quail. Poult. Sci. [Internet]. 2019; 98(5):2139–2149. doi: https://doi.org/gh7sc7 DOI: https://doi.org/10.3382/ps/pey568
Abd El–Hack ME, Abo ElMaati MF, Abusudah WF, Awlya OF, Almohmadi NH, Fouad W, Mohamed HS, Youssef IM, Al– Gabri NA, Othman SI, Allam AA, Taha AE, Tellez–Isaias G, Mansous AM. Consequences of dietary cinnamon and ginger oils supplementation on blood biochemical parameters, oxidative status, and tissue histomorphology of growing Japanese quails. Poult. Sci. [Internet]. 2024; 103(2):103314. doi: https://doi.org/p4z2 DOI: https://doi.org/10.1016/j.psj.2023.103314
Tatli Y, Olgun O. Rasyona lavanta esansiyel yaği ilavesinin yumurtlayan bildircinlarda performans, yumurta kalitesi ve serum parametreleri üzerine etkisi [Effect of supplementation of lavender essential oil to the diet on performance, egg quality and serum parameters in laying quails]. Bahri Dağdaş Hayvancılık Araştırma Derg. [Internet]. 2021 [cited Mar 24, 2025]; 10(1):20–27. Turkish. Available in: https://goo.su/bEHzfcz
Selmi S, Rtibi K, Grami D, Sebai H, Marzouki L. Lavandula stoechas essential oils protect against malathion–induces reproductive disruptions in male mice. Lipids Health Dis. [Internet]. 2018; 17(1):253. doi: https://doi.org/p4z3 DOI: https://doi.org/10.1186/s12944-018-0891-5
Li J, Yu Z, Han B, Li S, Lv Y, Wang X, Yang Q, Wu P, Liao Y, Qu B, Zhang Z. Activation of the GPX4/TLR4 signaling pathway participates in the alleviation of selenium yeast on deltamethrin– provoked cerebrum injury in quails. Mol. Neurobiol. [Internet]. 2022; 59(5):2946–2961. doi: https://doi.org/p4z4 DOI: https://doi.org/10.1007/s12035-022-02744-3
Ahmed–Farid OA, Rizk HA, Shehata AM. Hydrogen peroxide modulates redox status, energy metabolism, and gene expression in a dose–and time–dependent manner in rat liver. J. Biochem. Mol. Toxicol. [Internet]. 2018; 32(10):e22199. doi: https://doi.org/gdtzrs DOI: https://doi.org/10.1002/jbt.22199