Trace element levels in the brain tissue of red fox (Vulpes vulpes L.) in the Konya region
Abstract
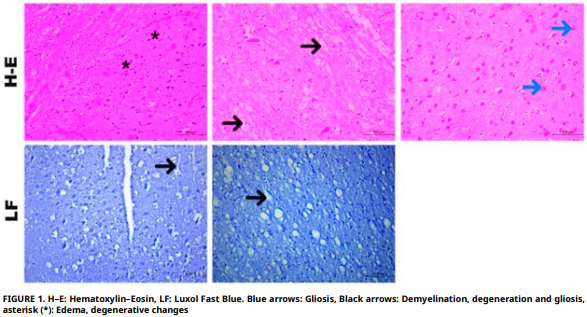
In nature, living beings serve and live as a link in the food chain in the ecosystem. Living beings in the wild are the last link in the food chain. They continue their existence by feeding on the nutritionally valuable foods they find in their natural habitats. People are leaving more residues in nature with developing technology. The effect of these residues is revealed by examining animals living in the wild. In this study, the determination, and histopathological examination of the concentration of trace elements in the brain tissue of red foxes (Vulpes vulpes) were carried out. In the study, the measurement of the elemental levels of trace elements manganese, iron, copper, zinc, and cobalt was carried out with the ICP–MS method. Since no other scientific article could be found on red foxes regarding manganese, iron, copper, zinc and cobalt, a comparison could not be made. Histopathologically, hyperemia, hemorrhage, gliosis, neuronal degeneration and necrosis, perivascular space expansion, perivascular inflammatory cell infiltration, demyelination, and perineural edema findings were observed in red fox brain tissue. A statistically significant correlation was detected between manganese and iron. This could be explained by exposure to shared environmental sources or a simultaneous role in brain metabolism. It is thought that such studies should be increased to ensure the continuity of red fox species in wildlife.
Downloads
References
Harris S. Distribution, habitat utilization and age structure of a suburban fox (Vulpes vulpes) population. Mammal Rev. [Internet]. 1977; 7(1):25–38. doi: https://doi.org/cx7vsp DOI: https://doi.org/10.1111/j.1365-2907.1977.tb00360.x
Kuru M. Omurgalı Hayvanlar [Vertebrate animals]. 5th ed. Ankara (Türkiye): Palme Yayıncılık; 1999. Turkish.
Demirsoy A. Yaşamın Temel Kuralları Omurgalılar/Amniyota (Sürüngenler, Kuşlar ve Memeliler) [The fundamental rules of life: Vertebrates–Amniota (Reptiles, Birds and Mammals)]. Volume III / Part II. 5th ed. Ankara (Türkiye): Meteksan Yayıncılık; 2003. Turkish.
Castañeda I, Doherty TS, Fleming PA, Stobo–Wilson AM, Woinarski JC, Newsome TM. Variation in red fox Vulpes vulpes diet in five continents. Mammal Rev. [Internet]. 2022; 52(3):328–342. doi: https://doi.org/gt2rtd DOI: https://doi.org/10.1111/mam.12292
Banfalvi, G. Heavy Metals, Trace elements and their cellular effects. In: Banfalvi G, editor. Cellular effects of heavy metals. Dordrecht (Netherlands): Springer; 2011. p. 3–28. DOI: https://doi.org/10.1007/978-94-007-0428-2_1
Wren CD. A review of metal accumulation and toxicity in wild mammals: I. Mercury. Environ. Res. [Internet]. 1986; 40(1):210–244. doi: https://doi.org/dftfs3 DOI: https://doi.org/10.1016/S0013-9351(86)80098-6
Lazarus M, Sekovanić A, Reljić S, Kusak J, Ferenčaković M, Sindičić M, Gomerčić T, Huber Đ. Lead and other trace element levels in brains of Croatian large terrestrial carnivores: influence of biological and ecological factors. Toxics [Internet]. 2022; 11(1):4. doi: https://doi.org/gwhvmg DOI: https://doi.org/10.3390/toxics11010004
Pandey G, Sharma M. Heavy metals causing toxicity in animals and fishes. Res. J. Anim. Vet. Fish. Sci. [Internet]. 2014 [cited Jun 2, 2025]; 2(2):17–23. Available in: https://goo.su/WRmYs DOI: https://doi.org/10.14737/journal.aavs/2014/2.4s.17.23
Wuana RA, Okieimen FE. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Not. [Internet]. 2011; 2011:402647. doi: https://doi.org/bv63sm DOI: https://doi.org/10.5402/2011/402647
Kumar S. Environmental contaminants and their impact on wildlife. In: Kumar S, editor. Toxicology and human health: Environmental exposures and biomarkers. Singapore (Singapore): Springer; 2023. p. 3–26. doi: https://doi.org/p78n DOI: https://doi.org/10.1007/978-981-99-2193-5_1
Kalisinska E, Lisowski P, Kosik–Bogacka DI. Red fox Vulpes vulpes (L., 1758) as a bioindicator of mercury contamination in terrestrial ecosystems of north–western Poland. Biol. Trace Elem. Res. [Internet]. 2012; 145:172–180. doi: https://doi.org/ckm338. DOI: https://doi.org/10.1007/s12011-011-9181-z
Garcês A, Pires I. Secrets of the astute red fox (Vulpes vulpes, Linnaeus, 1758): An inside–ecosystem secret agent serving One Health. Environments [Internet]. 2021; 8(10): 103. doi: https://doi.org/p8bn DOI: https://doi.org/10.3390/environments8100103
Heltai M, Markov G. Red fox (Vulpes vulpes, Linnaeus, 1758) as biological indicator for environmental pollution in Hungary. Bull. Environ. Contam. Toxicol. [Internet]. 2012; 89:910–914. doi: https://doi.org/gv46c8 DOI: https://doi.org/10.1007/s00128-012-0755-z
Mahler HR, Mackler B, Green DE, Bock RM. Studies on metalloflavoproteins III. Aldehyde oxidase: A molybdoflavoprotein. J. Biol. Chem. [Internet]. 1954; 210:465–480. doi: https://doi.org/p8bp DOI: https://doi.org/10.1016/S0021-9258(18)65471-7
Kalisińska E, Kot K, Łanocha–Arendarczyk N. Red fox as a potential bioindicator of metal contamination in a European environment. Chemosphere [Internet]. 2023; 319: 138037. doi: https://doi.org/gzwmtx DOI: https://doi.org/10.1016/j.chemosphere.2023.138037
Kwik–Uribe CL, Gietzen D, German JB, Golub MS, Keen CL. Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J. Nutr. [Internet]. 2000; 130(11):2821– 2830. doi: https://doi.org/gdwx DOI: https://doi.org/10.1093/jn/130.11.2821
Larkin EC, Ananda Rao G. Importance of fetal and neonatal iron: Adequacy for normal development of central nervous system. In: Dobbing J, editor. In: Brain, Behaviour, and Iron in the infant diet. London (UK): Springer–Verlag; 1990. p. 43–57. DOI: https://doi.org/10.1007/978-1-4471-1766-7_5
Clardy SL, Wang X, Zhao W, Liu W, Chase GA, Beard JL, Felt BT, Connor JR. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. In: Parvez H, Riederer P, editors. Oxidative stress and neuroprotection. Vienna (Austria): Springer–Verlag; 2006.p. 173–196. DOI: https://doi.org/10.1007/978-3-211-33328-0_19
Dallman PR. Biochemical basis for the manifestations of iron deficiency. Annu. Rev. Nutr. [Internet]. 1986; 6:13–40. doi: https://doi.org/dwpt32 DOI: https://doi.org/10.1146/annurev.nu.06.070186.000305
Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. [Internet]. 2015; 95(3):749–784. doi: https://doi.org/f7rp3g DOI: https://doi.org/10.1152/physrev.00035.2014
Bredholt M, Frederiksen LJ. Zinc in multiple sclerosis: A systematic review and meta–analysis. ASN Neuro. [Internet]. 2016; 8(3):1–9. doi: https://doi.org/gcdz4p DOI: https://doi.org/10.1177/1759091416651511
Tapiero H, Tew KD. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. [Internet]. 2003; 57(9):399–411. doi: https://doi.org/bmnfhf DOI: https://doi.org/10.1016/S0753-3322(03)00081-7
Takeda A. Zinc homeostasis and functions of zinc in the brain. Biometals [Internet]. 2001; 14:343–351. doi: https://doi.org/b8zgfv DOI: https://doi.org/10.1023/A:1012982123386
Prasad AS. Impact of the discovery of human zinc deficiency on health. J. Am. Coll. Nutr. [Internet]. 2009; 28(3):257–265. doi: https://doi.org/f3v8p8 DOI: https://doi.org/10.1080/07315724.2009.10719780
DiGirolamo AM, Ramirez–Zea M. Role of zinc in maternal and child mental health. Am. J. Clin. Nutr. [Internet]. 2009; 89(3):940S–945S. doi: https://doi.org/fwm7pc DOI: https://doi.org/10.3945/ajcn.2008.26692C
Kardos J, Héja L, Simon Á, Jablonkai I, Kovács R, Jemnitz K. Copper signalling: Causes and consequences. Cell. Commun. Signal. [Internet]. 2018; 16:71. doi: https://doi.org/ggb2f2 DOI: https://doi.org/10.1186/s12964-018-0277-3
Peña MM, Lee J, Thiele DJ. A delicate balance: Homeostatic control of copper uptake and distribution. J. Nutr. [Internet]. 1999; 129(7):1251–1260. doi: https://doi.org/gmvngx DOI: https://doi.org/10.1093/jn/129.7.1251
Kodama H, Fujisawa C. Copper metabolism and inherited copper transport disorders: Molecular mechanisms, screening, and treatment. Metallomics [Internet]. 2009; 1:42–52. https://doi.org/cxb596 DOI: https://doi.org/10.1039/B816011M
Kieliszek M, Bano I, Zare H. A comprehensive review on selenium and its effects on human health and distribution in middle eastern countries. Biol. Trace. Elem. Res. [Internet]. 2022; 200(3):971–987. doi: https://doi.org/p8bs DOI: https://doi.org/10.1007/s12011-021-02716-z
DeBenedictis CA, Raab A, Ducie E, Howley S, Feldmann J, Grabrucker AM. Concentrations of essential trace metals in the brain of animal species—a comparative study. Brain Sci. [Internet]. 2020; 10(7):460. doi: https://doi.org/p8bt DOI: https://doi.org/10.3390/brainsci10070460
Akcakavak G, Tuzcu M, Tuzcu N, Celik Z, Tural A, Dagar O. Investigation with Real–Time PCR and histopathology on the presence of H. felis, H. heilmannii and H. pylori in dogs. Rev. Cientif. FCV–LUZ. [Internet]. 2023; 33(1):1–7. doi: https://doi.org/mfsp DOI: https://doi.org/10.52973/rcfcv-e33214
Akcakavak G, Karatas O, Çelik Z, Tural A, Dağar O, Abduljabbar A, Kılınc B, Tuzcu M. Taxifolin attenuates cisplatin–induced kidney damage in rats via suppressing p53 and iNOS. J. Etlik Vet. Microbiol. [Internet]. 2024; 35(1):1–7. doi: https://doi.org/p8bv DOI: https://doi.org/10.35864/evmd.1458328
Laur N, Kinscherf R, Pomytkin K, Kaiser L, Knes O, Deigner HP. ICP–MS trace element analysis in serum and whole blood. PloS One. [Internet]. 2020; 15(5):e0233357. doi: https://doi.org/g823tq DOI: https://doi.org/10.1371/journal.pone.0233357
Nordic Committee on Food Analysis (NMKL). Trace elements – As, Cd, Hg, Pb and other elements: determination by ICP–MS after pressure digestion. Norway: NMKL; 2007.
Anon. MWS–2. Temassız Sıcaklık Ölçümüne Sahip Mikrodalga Parçalama Sistemi. V.3.0 Kullanma Talimatı [MWS–2. Microwave digestion system with non–contact temperature measurement. V.3.0 operating instructions]. Berghof Products + Instruments GmbH. 2004. Turkish.
Gutwerk D, Krämer R. Mikrowellenaufschlüsse steuern. Nachr. Chem. [Internet]. 2005; 53(10):1052–1053. doi: https://doi.org/ccsf5m DOI: https://doi.org/10.1002/nadc.20050531024
Plantin LO, Lying–Tunell U, Kristensson K. Trace elements in the human central nervous system studied with neutron activation analysis. Biol. Trace Elem. Res. [Internet]. 1987; 13:69–75. doi: https://doi.org/b7wdnd DOI: https://doi.org/10.1007/BF02796622
Jomova K, Makova M, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Rhodes CJ, Valko M. Essential metals in health and disease. Chem. Biol. Interact. [Internet]. 2022; 367:110173. doi: https://doi.org/gtb63p DOI: https://doi.org/10.1016/j.cbi.2022.110173
Mehri A. Trace Elements in Human Nutrition (II) An Update. Int. J. Prev. Med. [Internet]. 2020; 11:2. doi: https://doi.org/gt7r2f DOI: https://doi.org/10.4103/ijpvm.IJPVM_48_19
Pal A, Prasad R. Regional distribution of copper, zinc and iron in brain of Wistar rat model for non–Wilsonian brain copper toxicosis. Indian J. Clin. Biochem. [Internet]. 2016; 31:93–98. doi: https://doi.org/g7zf4n DOI: https://doi.org/10.1007/s12291-015-0503-3
Yakimoskii AF, Shantyr II, Vlasenko MA, Yakovleva MV. Effects of acyzol on zinc content in rat brain and blood plasma. Bull. Exp. Biol. Med. [Internet]. 2017; 162:293–294. doi: https://doi.org/p8bw DOI: https://doi.org/10.1007/s10517-017-3597-1
K ar atas O , Ak cak a v ak G. Det ermination b y immunohistochemistry of acute phase proteins in naturally infected sheep with listeriosis. Acta Vet. [Internet]. 2025; 75(1). doi: https://doi.org/p8bx DOI: https://doi.org/10.2478/acve-2025-0003
Lau G, Lai SH. Forensic histopathology. Forensic Pathol. Rev. [Internet]. 2009; 5:239–265. doi: https://doi.org/cqk55n DOI: https://doi.org/10.1007/978-1-59745-110-9_13
Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease. JAMA neurol. [Internet]. 2020; 77(8):1018–1027. doi: https://doi.org/ggx2kq DOI: https://doi.org/10.1001/jamaneurol.2020.2065
Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. [Internet]. 2014; 565:30–38. doi: https://doi.org/f22dr3 DOI: https://doi.org/10.1016/j.neulet.2013.12.071
Keaney J, Campbell M. The dynamic blood–brain barrier. FEBS J. [Internet]. 2015; 282(21):4067–4079. doi: https://doi.org/f7whcn DOI: https://doi.org/10.1111/febs.13412

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.


















