Efecto de Ankaferd Blood Stopper sobre el daño cardíaco inducido por cadmio en ratas macho
Resumen
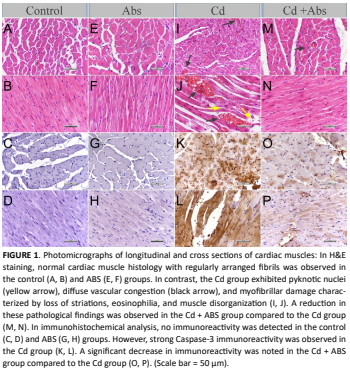
El cadmio (Cd), un contaminante ambiental ampliamente distribuido, es conocido por causar lesiones tisulares a través de mecanismos que implican estrés oxidativo, inflamación y apoptosis. El corazón es particularmente vulnerable a estos efectos tóxicos. Ankaferd Blood Stopper, un agente hemostático herbal estandarizado, también presenta notables actividades antioxidantes, antiinflamatorias y antiapoptóticas. Este estudio tuvo como objetivo explorar los posibles efectos protectores del Ankaferd Blood Stopper frente a la lesión cardíaca inducida por cadmio. Evaluar si la administración de Ankaferd Blood Stopper mitiga el daño histopatológico y apoptótico en el tejido cardíaco causado por la toxicidad por cadmio en ratas. Treinta y dos ratas macho Wistar Albino (350–450 g) se dividieron en cuatro grupos (n=8 cada uno): Control (solución salina, i.p.), Cd (2,5 mg/kg de CdCl₂, i.p.), Ankaferd Blood Stopper (0,5 mL de Ankaferd Blood Stopper, i.p.) y Cd + Ankaferd Blood Stopper (CdCl₂ + Ankaferd Blood Stopper, ambos i.p.). Tras el tratamiento, se recolectaron los tejidos cardíacos y se evaluaron mediante tinción histopatológica e inmunohistoquímica de Caspasa-3. También se obtuvieron muestras de sangre para análisis bioquímico. La exposición al cadmio provocó un daño miocárdico marcado, que incluyó infiltración inflamatoria, congestión vascular y fibras musculares desorganizadas. La expresión de Caspasa-3 confirmó un aumento de la actividad apoptótica. La administración de Ankaferd Blood Stopper redujo significativamente las lesiones histopatológicas y la inmunorreactividad de Caspasa-3 en el grupo Cd + Ankaferd Blood Stopper, lo que sugiere un efecto protector. El Ankaferd Blood Stopper atenuó de forma eficaz la lesión cardíaca inducida por cadmio, probablemente gracias a sus propiedades antioxidantes y antiinflamatorias. Estos hallazgos indican que el Ankaferd Blood Stopper podría ofrecer beneficios terapéuticos para prevenir o aliviar el daño cardiovascular inducido por metales pesados. Se recomienda realizar más investigaciones para validar estos efectos en modelos clínicos y a largo plazo.
Descargas
Citas
Gunes E. Antioxidant effects of Ankaferd Blood Stopper- doped polyvinyl pyrrolidone in an experimental model created in insects. Food Chem. Toxicol. [Internet]. 2021; 148:111935. doi: https://doi.org/p9hv DOI: https://doi.org/10.1016/j.fct.2020.111935
Ilhan I, Buyukbayram HI. Protective role of Ankaferd Blood Stopper on cadmium-induced acute nephrotoxicity. Med. J. SDU. [Internet]. 2023; 30(1):111-118. doi: https://doi.org/p9hw DOI: https://doi.org/10.17343/sdutfd.1239914
Ugur A, Sarac N, Cankal DA, Ozle M. The antioxidant and antimutagenic activities of Ankaferd Blood Stopper, a natural hemostatic agent used in dentistry. Turk. J. Med. Sci. [Internet]. 2016; 46(3):657-663. doi: https://doi.org/g9kbvr DOI: https://doi.org/10.3906/sag-1504-62
Arici E, Polat M. Determination of major heavy metal levels in pepper gas used as chemical agents in CBRN field. SDU J. Health Sci. [Internet]. 2024; 15(2):226-235. doi: https://doi.org/p9hz DOI: https://doi.org/10.22312/sdusbed.1480468
Ozbolat G, Tuli A. Effects of heavy metal toxicity on human health. Arch. Med. Rev. J. [Internet]. 2016; 25(4):502-521. doi: https://doi.org/p9h2 DOI: https://doi.org/10.17827/aktd.253562
Polat M, Ogut S. Heavy metals in some medicinal plants sold in herbal shops. Fresenius Environ. Bull. [Internet]. 2018 [cited 19 Jul 2025]; 27(4):1999-2002. Available in: https://goo.su/51tlgC
Jarup L. Hazards of heavy metal contamination. Br. Med. Bull. [Internet]. 2003; 68(1):167-182. doi: https://doi.org/cqwm49 DOI: https://doi.org/10.1093/bmb/ldg032
Gökdemir GS, Çakmak S, Demirtaş B, Gökdemir MT, Sogut O, Canpolat–Erkan RE, Aşır F, Yokus B. El efecto de la intoxicación aguda por monóxido de carbono en la necrosis cardíaca en ratas: en relación con los niveles de adiponectina. Rev. Cient. FCV-LUZ. [Internet]. 2025; 35(1):8. doi: https://doi.org/p9h4 DOI: https://doi.org/10.52973/rcfcv-e35532
Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol. [Internet]. 2005; 204(3):274-308. doi: https://doi.org/cpg8kx DOI: https://doi.org/10.1016/j.taap.2004.09.007
Joseph P. Mechanisms of cadmium carcinogenesis. Toxicol. Appl. Pharmacol. [Internet]. 2009; 238(3):272-279. doi: https://doi.org/cwd3gk DOI: https://doi.org/10.1016/j.taap.2009.01.011
Aktop S, EmekliAlturfan E, Ozer C, Gonul O, Garip H, Yarat A, Goker K. Effects of Ankaferd Blood Stopper and Celox on the tissue factor activities of warfarin-treated rats. Clin. Appl. Thromb. Hemost. [Internet]. 2013; 20(1):1621. doi: https://doi.org/f5kmzh DOI: https://doi.org/10.1177/1076029613490254
Bell RR, Nonavinakere VK, Soliman MR. Intratracheal exposure of the guinea pig lung to cadmium and/ or selenium: a histological evaluation. Toxicol. Lett. [Internet]. 2000; 114(1-3):101-109. doi: https://doi.org/b2cq2n DOI: https://doi.org/10.1016/S0378-4274(99)00286-6
Gulle K, Ceri NG, Akpolat M, Arasli M, Demirci B. The effects of dexpanthenol in streptozotocin-induced diabetic rats: histological, histochemical and immunological evidences. Histol. Histopathol. [Internet]. 2014 [cited 14 Jul 2025]; 29(10):1305-1313. Available in: https://goo.su/IenUcR
Skalli O, Gabbiani G. The biology of the myofibroblast relationship to wound contraction and fibrocontractive disease. In: Clark RAF, Henson PM, editors. The Molecular and Cellular Biology of Wound Repair. New York: Plenum Press; 1988. p. 373-402. DOI: https://doi.org/10.1007/978-1-4615-1795-5_17
Nohl H. Generation of superoxide radicals as byproducts of cellular respiration. Ann. Biol. Clin. (Paris). 1994; 52(3):199-204.
Senel O, Cetinkale O, Ozbay G, Ahcioglu F, Bulan R. Oxygen free radicals impair wound healing in ischemic rat skin. Ann. Plast. Surg. [Internet]. 1997; 39(5):516-523. doi: https://doi.org/b62xjd DOI: https://doi.org/10.1097/00000637-199711000-00012
Agaev AIu, Nikolaev AV, Abasov BKh, Mamedov LA, Zakharov VV, Bashirov EA, Bagirov GS. Antioxidant therapy of purulent wounds in animal experiments. Bull. Exp. Biol. Med. 1989; 108(7):35-37. DOI: https://doi.org/10.1007/BF00839774
Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species, and tissue damage. Curr. Pharm. Des. [Internet]. 2004; 10(14):1611-1626. doi: https://doi.org/fctrhh DOI: https://doi.org/10.2174/1381612043384664
Salvi A, Battaglia V, Brunati AM,La Rocca N, Tibaldi E, Pietrangeli P, Marcocci L, Mondovì B, Rossi CA, Toninello A. Catalase takes part in rat liver mitochondria oxidative stress defense. J. Biol. Chem. [Internet]. 2007; 282(33):24407-24415. doi: https://doi.org/cfmtms DOI: https://doi.org/10.1074/jbc.M701589200
Attia AA, El-Mazoudy RH, El-Shenawy NS. Antioxidant role of propolis extract against oxidative damage of testicular tissue induced by insecticide chlorpyrifos in rats. Pestic. Biochem. Physiol. [Internet]. 2012; 103(2):87-93. doi: https://doi.org/mh2g DOI: https://doi.org/10.1016/j.pestbp.2012.04.002
Ozen T, Korkmaz H. Modulatory effect of Urtica dioica L. (Urticaceae) leaf extract on biotransformation enzyme systems, antioxidant enzymes, lactate dehydrogenase and lipid peroxidation in mice. Phytomedicine. [Internet]. 2003; 10(5):405-415. doi: https://doi.org/dzszb9 DOI: https://doi.org/10.1078/0944-7113-00275
Rezg R, Mornagui B, Kamoun A, El-Fazaa S, Gharbi N. Effect of subchronic exposure to malathion on metabolic parameters in the rat. C. R. Biol. [Internet]. 2007; 330(2):143-147. doi: https://doi.org/cpp239 DOI: https://doi.org/10.1016/j.crvi.2006.11.002
Romeu M, Mulero M, Giralt M, Folch J, Nogués MR, Torres A, Fortuño A, Sureda FX, Cabré M, Paternáin JL, Mallol J. Parameters related to oxygen free radicals in erythrocytes, plasma, and epidermis of the hairless rat. Life Sci. [Internet]. 2002; 71(15):1739-1749. doi: https://doi.org/fwhd5s DOI: https://doi.org/10.1016/S0024-3205(02)01946-X
Tuthill DD, Bayer V, Gallagher AM, Drohan WN, MacPhee MJ. Assessment of topical hemostats in a renal hemorrhage model in heparinized rats. J. Surg. Res. [Internet]. 2001; 95(2):126-132. https://doi.org/d7c5j7 DOI: https://doi.org/10.1006/jsre.2000.6027
Elg M, Gustafsson D, Carlsson S. Antithrombotic effects and bleeding time of thrombin inhibitors and warfarin in the rat. Thromb. Res. [Internet]. 1999; 94(3):187-197. doi: https://doi.org/d86rkp DOI: https://doi.org/10.1016/S0049-3848(98)00213-8
Merian E. Metals and their compounds in the environment: Occurrence, analysis, and biological relevance. Weinheim: VCH; 1991.
Appleton J, Lee KM, Sawicka-Kapusta K, Damek M, Cooke M. Heavy metal content in the teeth of bank vole (Clethrionomys glareolus) as an exposure marker of environmental pollution in Poland. Environ. Pollut. [Internet]. 2000; 110(3):441-449. doi: https://doi.org/cksr8d DOI: https://doi.org/10.1016/S0269-7491(99)00318-8
Kjellstrom T, Elinder CG, Friberg L. Conceptual problems in establishing the critical concentration of cadmium in human kidney cortex. Environ. Res. [Internet]. 1984; 33(2):284-295. doi: https://doi.org/cgbhnb DOI: https://doi.org/10.1016/0013-9351(84)90025-2
Sangartit W, Kukongviriyapan U, Donpunha W, Pakdeechote P, Kukongviriyapan V, Surawattanawan P, Greenwald SE. Tetrahydrocurcumin protects against cadmium-induced hypertension, raised arterial stiffness and vascular remodeling in mice. PLoS One. [Internet]. 2014; 9(12):e114908. doi: https://doi.org/f6xb8d DOI: https://doi.org/10.1371/journal.pone.0114908
Saleh RM, Awadin WF. Biochemical and histopathological changes of subacute cadmium intoxication in male rats. Environ. Sci. Pollut. Res. [Internet]. 2017; 24(32):25475- 25481. doi: https://doi.org/gckjns DOI: https://doi.org/10.1007/s11356-017-0348-9
Bhattacharjee B,PalP K, Ghosh A K,Mishra S, Chattopadhyay A, Bandyopadhyay D. Aqueous bark extract of Terminalia arjuna protects against cadmiuminduced hepatic and cardiac injuries in male Wistar rats through antioxidative mechanisms. Food Chem. Toxicol. [Internet]. 2019; 124:249264. doi: https://doi.org/gqxjrm DOI: https://doi.org/10.1016/j.fct.2018.12.008
Li Y, Li H, Xiao L, Zhou L, Shentu J, Zhang X, Fan J. Hemostatic efficiency and wound healing of natural zeolite granules in lethal rabbit model of complex groin injury. Materials. [Internet]. 2012; 5(12):2586-2595. doi: https://doi.org/f4h5zj DOI: https://doi.org/10.3390/ma5122586
Tucovic D, Popov-Aleksandrov A, Mirkov I, Ninkov M, Kulas J, Zolotarevski L, Vukojevic V, Mutic J, Tatalovic N, Kataranovski M. Oral cadmium exposure affects skin immune reactivity in rats. Ecotoxicol. Environ. Saf. [Internet]. 2018; 164:12-20. doi: https://doi.org/gfn3vc DOI: https://doi.org/10.1016/j.ecoenv.2018.07.117
Halliwell B, Hoult JR, Blake DR. Oxidants, inflammation, and anti-inflammatory drugs. FASEB J. [Internet]. 1988; 2(13):2867-2873. doi: https://doi.org/p9jg DOI: https://doi.org/10.1096/fasebj.2.13.2844616
Cheel J, Van Antwerpen P, Tůmová L, Onofre G, Vokurková D, ZouaouiBoudjeltia K, Vanhaeverbeek M, Nève J. Free radical-scavenging, Antioxidant and immunostimulating effects of licorice infusion (Glycyrrhiza glabra L.). Food Chem. [Internet]. 2010; 122(3):508-517. doi: https://doi.org/cvcgx8 DOI: https://doi.org/10.1016/j.foodchem.2010.02.060
Hong YK, Wu HT, Ma T, Liu WJ, He XJ. Effects of Glycyrrhiza glabra polysaccharides on immune and antioxidant activities in high-fat mice. Int. J. Biol. Macromol. [Internet]. 2009; 45(1):61-64. doi: https://doi.org/c67665 DOI: https://doi.org/10.1016/j.ijbiomac.2009.04.001